
If you label a carbon with a C, you also must draw in the hydrogens for that carbon. Sometimes, one or more carbon atoms in a line structure will be depicted with a capital C, if doing so makes an explanation easier to follow. Our image to structure conversion is perfect for.
Skeletal structure chemistry calculator full#
Conversely, very small molecules such as ethane should be drawn with their full Lewis or condensed structures. No need to draw a chemical structure by yourself Easily convert images to ChemDraw with Mathpix Snip. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. Figure 2. Comparison between Lewis structure and line structure.Īs you can see, the ‘pared down’ line structure makes it much easier to see the basic structure of the molecule and the locations where there is something other than C-C and C-H single bonds. Skeletal ormulas imply a carbon atom at the corners and ends of lines. Hydrogens bonded to nitrogen, oxygen, sulfur, or anything other than carbon are shown, but are usually drawn without showing the bond. The following examples illustrate the convention. Condensed structural chemical formulas show the hydrogen atoms (or other atoms or groups) right next to the carbon atoms to which they are attached. Hydrogens attached to carbons are generally not shown: rather, like lone pairs, they are simply implied (unless a positive formal charge is shown, all carbons are assumed to have a full octet of valence electrons). Open-chain molecules are usually drawn out in a ‘zig-zig’ shape.
Skeletal structure chemistry calculator free#
Carbon atoms are depicted not by a capital C, but by a ‘corner’ between two bonds, or a free end of a bond. The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. 8 Two chemical tests are carried out on an aqueous solution of an aromatic. More commonly, organic and biological chemists use an abbreviated drawing convention called line structures, also known as skeletal structures or line bond structures. Determine the Lewis Structure for each of these household chemicals.

When you do this, you will see the two CH must be double bonded.Ĭommon organic compounds that you likely have at home are: acetone (CH 3COCH 3) found in nail polish remover, acetic acid (CH 3COOH) found in vinegar, and isopropanol ((CH 3) 2CHOH) found in rubbing alcohol. Always double check your structure to ensure every carbon is making four bonds. The COOH represent a carboxylic acid, which means you have a C=O connected to an O-H. The (CH 2) 7 represents a repeating unit, meaning you must draw seven CH 2‘s one after another, which are bonded to a CH which is bonded to a CH, and then another seven CH 2‘s.
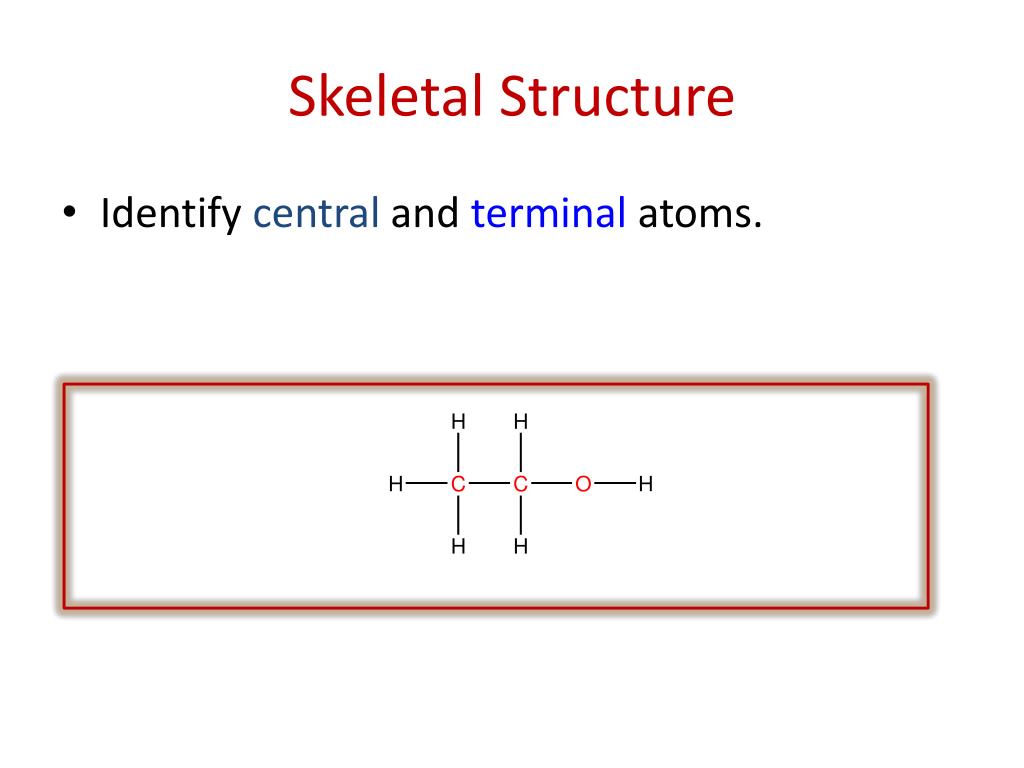
There are three carbons, one, two, three. So on the left here is one possible Lewis dot structure that you can draw that has that molecular formula. Determine the Lewis Structure of the following condensed structure of oleic acid, a fatty acid that is found naturally in various animal and vegetable fats and oils. Lets say were given the molecular formula C three H eight O, and were asked to draw a Lewis dot structure.


 0 kommentar(er)
0 kommentar(er)
